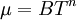Power-law viscosity law
From CFD-Wiki
(Difference between revisions)
Jola (Talk | contribs)
(New page: A power-law can be used as an approximation of the viscosity of dilute gases. For dilute gases at moderate temperatures, this form is slightly less accurate than Sutherland's law. The ...)
Newer edit →
(New page: A power-law can be used as an approximation of the viscosity of dilute gases. For dilute gases at moderate temperatures, this form is slightly less accurate than Sutherland's law. The ...)
Newer edit →
Revision as of 21:17, 17 May 2007
A power-law can be used as an approximation of the viscosity of dilute gases. For dilute gases at moderate temperatures, this form is slightly less accurate than Sutherland's law. The power-law viscosity law can be written as:
Where 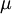 is the viscosity in kg/m-s,
is the viscosity in kg/m-s, 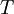 is the static temperature in K, and
is the static temperature in K, and 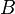 is a dimensional coefficient. For air at moderate temperatures and pressures
is a dimensional coefficient. For air at moderate temperatures and pressures 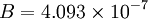 , and
, and 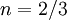 .
.
The power-law viscosity law can also be written as:
Where 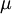 is the viscosity in kg/m-s,
is the viscosity in kg/m-s, 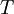 is the static temperature in K,
is the static temperature in K, 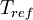 is a reference value in K,
is a reference value in K, 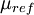 is a reference value in kg/m-s. For air at moderate temperatures and pressures,
is a reference value in kg/m-s. For air at moderate temperatures and pressures, 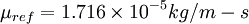 ,
, 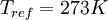 , and
, and 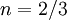 .
.
Note that there exists a different power-law for non-Newtonian fluids!